
At energies above 50 eV, the sum of ECS and ICS for the investigated targets is in satisfactory agreement with the respective measured TCS. Additionally, the integral elastic cross section (ECS) and ionization cross section (ICS) have been calculated from intermediate to high electron-impact energies using model methods.

To examine the role of permethylation in the scattering, the measured TCS energy functions for X(CH3)4 compounds (X = C, Si, Ge) have been compared to the TCS curves for XH4 molecules.
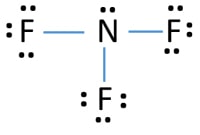
We attributed the TCS features to the resonant processes involved in the electron-molecule scattering. Additional weak structures are also located at higher electron energies. The measured TCS energy dependences show very pronounced broad enhancement, peaking near 5.5 eV for Si(CH3)4 and Ge(CH3)4 molecules and around 6.5 eV for C(CH3)4.

It was also found that the etch rate strongly depends on the wafer position on the processing chamber electrode, and that the etch selectivity reached 96–131 in the discharge conditions of RF powers (3730–4180 W) and gas pressures (3.6–4 Torr).Ībsolute grand-total cross sections (TCSs) for electron scattering from tetramethylmethane, tetramethylsilane, and tetramethylgermane molecules have been measured at electron-impact energies extending from around 0.5 to 300 eV in the linear electron-transmission experiment. The etch rate of the silicon films significantly increases while the silicon oxide is slightly etched with the gas pressures and powers. In this study, plasma density measurements were performed near the plume region of the remote plasma source (RPS) in Ar/$).$ We also performed downstream etching of silicon and silicon oxide films in this system. The TCS for NF3 is also compared with the TCS for ammonia (NH3) which was supplementary measured and the effect of substitution of fluorine atoms for hydrogen (perfluorination effect) is demonstrated and discussed. The sum of calculated cross sections reasonably reproduces the intermediate-energy experimental TCS, with respect to the shape and value. The cross section for ionization has been computed as well using the binary-encounter-Bethe approach.
#NF3 MOLECULAR GEOMETRY PLUS#
The integral elastic cross section has been calculated at intermediate energies using an independent atom method with a static plus polarization model potential. The low-energy enhancement is superimposed with some weak features located near 1.8, 2.2, and 2.8 eV. It was found that the TCS energy function for NF3 is dominated with two pronounced enhancements: one resonantlike centered between 2 and 3 eV with the maximum value of 28×10−20 m2 followed with a minimum at around 7–12 eV(∼17×10−20 m2), and the second much broader enhancement located around 40 eV (19×10−20 m2 in the maximum). Going back to your question, we are supposed to compare $\ce$ also.Absolute total cross sections (TCS’s) for 0.5–370-eV electrons scattered by nitrogen trifluoride (NF3) molecules have been measured using a linear transmission method under single collision conditions. You can think of how s orbitals are spherical in shape, and a large s character would lead to more "spherical" hybrid orbitals, and that would lead to larger bond angles. What has all of this got to do with bond angles? Well, more s character leads to larger bond angles. Thus, Bent's rule can just as easily be formulated as follows: p character concentrates in orbitals directed towards electronegative substituents (the central atom doesn't have to waste its low energy s orbitals, the orbitals on the electronegative atom, can (possibly) take care of it. Which is why molecules like to use them (s orbitals) in bonds where there is "more" electron density to stabilise. Since s orbitals are lower in energy than p orbitals, they are better at stabilising electrons. Before that, I'll note that we concern ourselves with the hybridisation of the orbitals at the central atom. What follows below is a crude explanation. This can be argued on the basis of Bent's rule concisely statedĪtomic s character concentrates in orbitals directed toward electropositive substituents


 0 kommentar(er)
0 kommentar(er)
